Growth of a TiO2 nanotubular layer without presence of nanograss in a short time
DOI:
https://doi.org/10.31349/RevMexFis.65.49Keywords:
TiO2, nanotube, nanograss, anatase, anodization, Raman, SEMAbstract
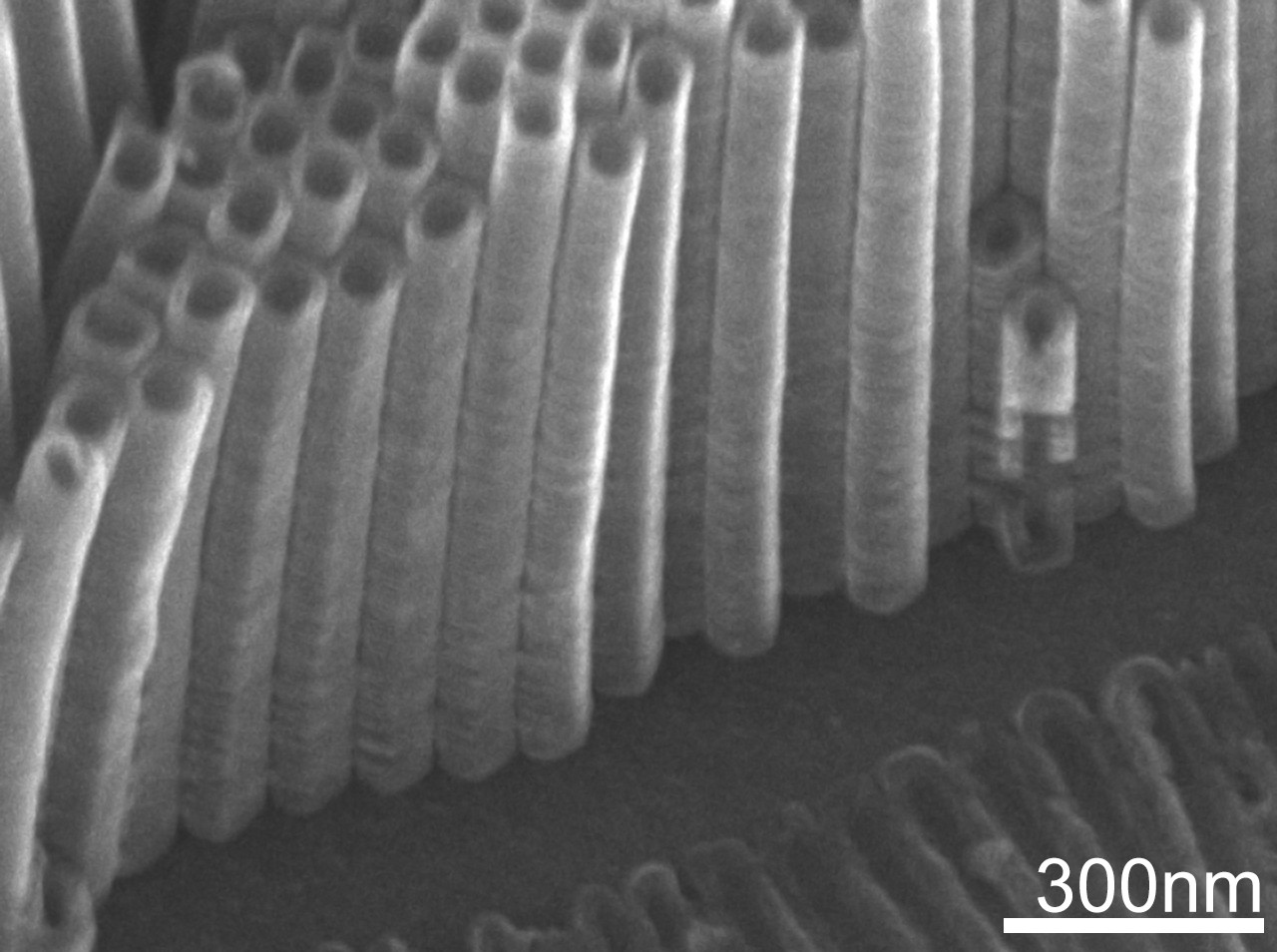
Many studies, focused in TiO2 anodized, uses frequently a NH4F salt concentration from 0.3 – 0.5 wt% and the whole information about how voltage, time and even pH affects to nanotubes morphology, are effective just for these concentration range. It is known, increasing salt concentration, the electrolyte increases their conductivity and anodization speed (oxidation-dissolution) suffer also an increment and for a specifically concentration 1.2wt%, there is no data about morphology repercussions. A TiO2 nanotubular matrix is synthesized, in order to identify the range of time where it is possible to obtain with no presence of nanograss. The anodization process consists of an organic electrolyte of ethylene glycol, deionized water and 1.2 wt% NH4F salts, constant potential of 30 V and a time lapse from 10 to 60 minutes (short time). All anodized samples are rinsed and annealed to 400 °C by 4 hours to obtain an anatase crystalline structure; no samples are cleaned in ultrasonic bath to preserve the nanograss structure. Optical characterization was performed by Raman Spectroscopy to identify the increases in signal intensity, associated with thickness. The morphological characterization was carried out by Scanning Electron Microscopy to verify the presence and density of the nanograss and nanotubes.
Downloads
Published
How to Cite
Issue
Section
License
Authors retain copyright and grant the Revista Mexicana de Física right of first publication with the work simultaneously licensed under a CC BY-NC-ND 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.

